Processing of blast furnace collector dusts for their utilization in the steel industry
DOI:
https://doi.org/10.37636/recit.v7n3e277Keywords:
Blast furnace, Collector dusts, Leaching, Magnetic separation, Powder processingAbstract
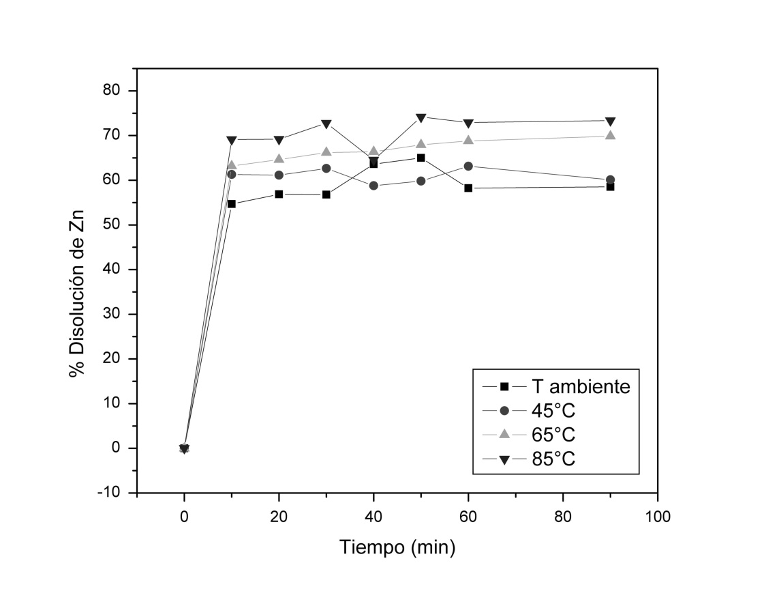
The production of pig iron involves the generation of by-products such as collector dusts, which are attractive due to their Fe content. However, they cannot be directly reused because of their high content of Zn and alkalis. Therefore, this research aims to explore their utilization through acid leaching. To achieve this, a sample was processed through grinding (1h) and low-intensity magnetic separation, and then the effect of hydrochloric acid as a leaching agent was evaluated at each stage of the process (0.10, 0.25, 0.50 and 1.0M HCl). Furthermore, the effect of temperature was analyzed for the system with 1M HCl. The results indicate that working with systems with high HCl concentration in both fresh and ground material made it possible to dissolve up to 65% Zn content in the initial sample (with 38.8% Fe). On the other hand, leaching the magnetically treated dusts achieved Zn solutions of 56% and 65% for 1 and 2 cleaning cycles, respectively. Regarding the Fe content, an increase was observed compared to the initial content, reaching from 53.8% to 59% Fe (making its processing feasible). Furthermore, as the temperature increased, the leaching of Zn was privileged, reaching up to 74% dissolution at 85°C. In the case of Na2O, P, and K2O dissolution, an increase in working temperature accelerated the dissolution kinetics. However, in the case of Fe, an increase in working temperature led to a decrease in its concentration. Lastly, thermodynamic analysis determined the ∆G° values of the reactions, indicating their spontaneity, i.e., they occur without the need of applied energy. In conclusion, it was possible to compare the effect of leaching systems at each stage of processing. At room temperature, leaching of the untreated dust achieved up to 65% Zn dissolution with 38.8% Fe, and after 2 rounds of magnetic cleaning, a dissolution of 65% Zn with a 61.4% Fe content is attained. By increasing the temperature, most of the reactions involved in the leaching process are catalyzed, especially the zinc dissolution (up to 74%). The feasibility of the involved reactions can be supported through thermodynamics.
Downloads
References
I. Cameron, L. Sukhram, K. Lefebvre and W. Davenport, Blast Furnace Ironmaking: Analysis, Control, and Optimization, (Edition unavailable), Elsevier Science, 2019. [E-book] Available: https://doi.org/10.1016/B978-0-12-814227-1.00061-0 DOI: https://doi.org/10.1016/B978-0-12-814227-1.00061-0
P. Li, G. Wang, Y. Dong and Y. Zhuo, “A Review on Desulfurization Technologies of Blast Furnace Gases,” Curr. Pollut. Rep., vol. 8, no. 2, pp. 189–200, 2022. https://doi.org/10.1007/s40726-022-00212-z DOI: https://doi.org/10.1007/s40726-022-00212-z
M. Oge, D. Ozkan, M. B. Celik, M. S. Gok and A. C. Karaoglanli, “An Overview of Utilization of Blast Furnace and Steelmaking Slag in Various Applications”, Mater. Today: Proc., vol. 11, no. 1, pp. 516-525, 2019. https://doi.org/10.1016/j.matpr.2019.01.023. DOI: https://doi.org/10.1016/j.matpr.2019.01.023
C. Hamann, M. Spanka, D. Stolle, G. Auer, E. Weingart, D. Al-Sabbagh, M. Ostermann and C. Adam, “Recycling of blast-furnace sludge by thermochemical treatment with spent iron(II) chloride solution from steel pickling,” J. Hazard. Mater., vol. 402, 2020. https://doi.org/10.1016/j.jhazmat.2020.123511 DOI: https://doi.org/10.1016/j.jhazmat.2020.123511
R. Ochoa and M. Farfan, “Blast furnace dust: An alternative for the improvement of granular material for pavements. J. Phys. Conf. Ser., vol.1386, 2019. https://doi.org/10.1088/1742-6596/1386/1/012033 DOI: https://doi.org/10.1088/1742-6596/1386/1/012033
X. Yang, B. Xie, F. Wang, P. Ning, K. Li, L. Jia, J. Feng, F. Xia, “Resource utilization of hazardous solid waste blast furnace dust: Efficient wet desulfurization and metal recovery,” Chemosphere, vol. 314, pp. 137592. 2023. https://doi.org/10.1016/j.chemosphere.2022.137592 DOI: https://doi.org/10.1016/j.chemosphere.2022.137592
Y. Takahashi and S. Suntharalingam, “Experimental Study on Autogenous Shrinkage Behaviors of Different Portland Blast Furnace Slag Cements,” Constr. Build. Mater., vol. 230, 2020. https://doi.org/10.1016/j.conbuildmat.2019.116980 DOI: https://doi.org/10.1016/j.conbuildmat.2019.116980
L. Ding, W Ning, Q. Wang, D. Shi and L. Luo, “Preparation and characterization of glass-ceramic foams from blast furnace slag and waste glass,” Mater. Lett., vol. 141, pp. 327-329, 2015. https://doi.org/10.1016/j.matlet.2014.11.122 DOI: https://doi.org/10.1016/j.matlet.2014.11.122
H. A. Rondón, J. C. Ruge, D. F. Patiño, H. A. Vacca, F. A. Reyes and M. Muniz, “Blast furnace slag as a substitute for the fine fraction of aggregates in an asphalt mixture,” J. Mater. Civ. Eng., vol. 30, no. 10, 2018. https://doi.org/10.1061/(ASCE)MT.1943-5533.0002409 DOI: https://doi.org/10.1061/(ASCE)MT.1943-5533.0002409
R. Mahakhud, M. Priyadarshini and J. Prakash, “Utilization of ground granulated blast-furnace slag powder in brick industry: A new generation building material,” Mater. Today: Proc., 2023. https://doi.org/10.1016/j.matpr.2023.03.707 DOI: https://doi.org/10.1016/j.matpr.2023.03.707
X. Yin, C. M. Zhang, Y. H. Cai, C. M. Zhao, J. Yang and B. Li, “Molten slag bath reduction: Carbon-thermal reduction of blast furnace dust in molten blast furnace slag,” IOP Conf. Ser.: Mater. Sci. Eng., vol. 230, no. 1, pp. 012027, 2017. https://dx.doi.org/10.1088/1757-899X/230/1/012027 DOI: https://doi.org/10.1088/1757-899X/230/1/012027
G. Zhao, R. Li, X. Xing, J. Ju, X. Li, J. Zu, “Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process,” Green Process. Synth., vol.12, no. 1, pp. 20230045, 2023. https://doi.org/10.1515/gps-2023-0045 DOI: https://doi.org/10.1515/gps-2023-0045
X. Liu, Z. Liu, J. Zhang and X. Xing, "Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction," High Temp. Mater. Process., vol. 38, no. 2019, pp. 767-772, 2019. https://doi.org/10.1515/htmp-2019-0023 DOI: https://doi.org/10.1515/htmp-2019-0023
S. Mustafa, L. Luo, B.-T. Zheng, C.-X. Wei and N. Christophe, “Effect of Lead and Zinc Impurities in Ironmaking and the Corresponding Removal Methods: A Review,” Metals, vol. 11, no. 3, pp. 407, 2021. https://doi.org/10.3390/met11030407 DOI: https://doi.org/10.3390/met11030407
A. Andersson, H. Ahmed, J. Rosenkranz, C. Samuelsson and B. Bjorkman, “Characterization and upgrading of a low Zinc-containing and fine blast furnace sludge – a multi objective analysis,” ISIJ Int., vol. 57, no. 2, pp. 262-271, 2017. https://doi.org/10.2355/isijinternational.ISIJINT-2016-512 DOI: https://doi.org/10.2355/isijinternational.ISIJINT-2016-512
I. O. Acuña, J. A. López and A. Perea, “Lixiviación de Zn a partir de polvo del colector del alto horno para su aplicación potencial como material reciclado,” CienciAcierta Revista científica, tecnológica y humanística, no. 62, 2020.
N. Nayak, “Characterization of blast furnace flue dust- an assessment for its utilization,” Mater. Today: Proc., vol.50, no. 2, pp. 2078-2083, 2021. https://doi.org/10.1016/j.matpr.2021.09.417 DOI: https://doi.org/10.1016/j.matpr.2021.09.417
K. Brunelli and M. Dabalá, “Ultrasound effects on zinc recovery from EAF dust by sulfuric acid leaching,” Int. J. Miner., Metall. Mater., vol. 22, no. 4, pp. 353-362, 2015. https://doi.org/10.1007/s12613-015-1080-4 DOI: https://doi.org/10.1007/s12613-015-1080-4
L. Hernández, I. Daza, G. Amaral, F. Beneduce and G. Lenz, “Microstructural, thermochemistry and mechanical evaluation of self-reducing pellets using electric arc furnace (EAF) dust containing zinc for Waelz process,” Matéria, vol. 23, no. 2, 2018. https://doi.org/10.1590/S1517-707620180002.0343 DOI: https://doi.org/10.1590/s1517-707620180002.0343
P. Dvorak and H. Vu, “Zinc recovery from flue dust,” Journal of the polish mineral engineering society, vol. 18, pp. 195-198, 2017. https://doi.org/10.29227/IM-2017-01-31
Y. Xue, X. Hao, X. Liu, and N. Zhang, “Recovery of Zinc and Iron from Steel Mill Dust—An Overview of Available Technologies,” Materials, vol. 15, no. 12, pp. 4127, 2022. https://doi.org/10.3390/ma15124127 DOI: https://doi.org/10.3390/ma15124127
H. S. Lee, D. Park, Y. Hwang, J. Ha, H. Shin, “Toward high recovery and selective leaching of zinc from electric arc furnace dust with different physicochemical properties,” Environ. Eng. Res., vol. 25, no. 3, pp. 335-344, 2019. https://doi.org/10.4491/eer.2019.132 DOI: https://doi.org/10.4491/eer.2019.132
K. Binnemans, P. T. Jones, P.T., A. Fernández and V. Torres “Hydrometallurgical Processes for the Recovery of Metals from Steel Industry By-Products: A Critical Review,” J. Sustain. Metall., vol. 6, pp. 505–540 (2020). https://doi.org/10.1007/s40831-020-00306-2 DOI: https://doi.org/10.1007/s40831-020-00306-2
F. Kukurugya, T. Vindt, and T. Havlík, “Behavior of zinc, iron and calcium from electric arc furnace (EAF) dust in hydrometallurgical processing in sulfuric acid solutions: Thermodynamic and kinetic aspects,” Hydrometallurgy, vol. 154, pp. 20–32, 2015. https://doi.org/10.1016/j.hydromet.2015.03.008 DOI: https://doi.org/10.1016/j.hydromet.2015.03.008
R. Kusumaningrum, A. Fitroturokhmah, G. Sinaga, A. Wismogroho, W. Widayatno, L. J. Diguna and Amal, Muhamad, “Study: leaching of zinc dust from electric arc furnace waste using oxalic acid,” IOP Conf. Ser. Mater. Sci. Eng., vol. 478, no. 2019, pp. 012015, 2019. https://doi.org/10.1088/1757-899X/478/1/012015 DOI: https://doi.org/10.1088/1757-899X/478/1/012015
M. J. Soria, G. I. Davila, F. R. Carrillo, A. A. Gonzalez, N. Picazo, F. J. Lopez, J. Ramos, “Oxidative Leaching of Zinc and Alkalis from Iron Blast Furnace Sludge,” Metals, vol. 9, no. 9, pp. 1015, 2019. https://doi.org/10.3390/met9091015 DOI: https://doi.org/10.3390/met9091015
Y. Y. Teo, H. S. Lee, Y. C. Low, S. W. Choong and K. O. Low, “Hydrometallurgical extraction of zinc and iron from electric arc furnace dust (EAFD) using hydrochloric acid,” J. Phys. Sci., vol. 29, no. 3, pp. 49–54, 2018. https://doi.org/10.21315/jps2018.29.s3.6
S. Langová, J. Lesko and D. Matysek, “Selective leaching of zinc from zinc ferrite with hydrochloric acid,” Hydrometallurgy, vol. 95, no. 179-182, 2009. https://doi.org/10.1016/j.hydromet.2008.05.040 DOI: https://doi.org/10.1016/j.hydromet.2008.05.040
M. Irannajad, M. Meshkini, A. R. Azadmehr, “Leaching of zinc from low grade oxide ore using organic ACID,” Physicochem. Probl. Miner. Process., vol. 49, no. 2, pp. 547-555, 2013. https://doi.org/10.21315/jps2018.29.s3.6 DOI: https://doi.org/10.21315/jps2018.29.s3.6
J. Borda and R. Torres, “Comparative study of selective zinc leaching from EAFD using carboxylic agents,” Rev. Mex. Ing. Quím., vol. 20, no. 1, 2021. https://doi.org/10.24275/rmiq/IA2022 DOI: https://doi.org/10.24275/rmiq/IA2022
R. Larba, I. Boukerche, N. Alane, N. Habbache, S. Djerad and L. Tifouti, “Citric acid as an alternative lixiviant for zinc oxide dissolution,” Hydrometallurgy, vol. 134, pp. 117–123, 2013. https://doi.org/10.1016/j.hydromet.2013.02.002 DOI: https://doi.org/10.1016/j.hydromet.2013.02.002
R. Baidya, S. Kumar and U. Parlikar, “Blast furnace flue dust co-processing in cement kiln - A pilot study,” Waste Manag. Res., vol. 37, no. 3, pp. 261–267, 2019. https://doi.org/10.1177/0734242X18816791 DOI: https://doi.org/10.1177/0734242X18816791
B. Das, S. Prakash, P. Reddy, S. Biswal, B. Mohapatra and V. Misra, “Effective utilization of blast furnace flue dust of integrated steel plants,” The European Journal of Mineral Processing and Environmental Protection, vol. 2, no. 2, pp. 61-68, 2002.
A. Yehia and F. El-Rehiem, “Recovery and utilization of iron and carbon values from blast furnace flue dust,” Miner. Process. Extr. Metall., vol. 114, no. 4, pp. 207-211, 2005. https://doi.org/10.1179/037195505X28519 DOI: https://doi.org/10.1179/037195505X28519
J. Ju, Y. Feng, H. Li, Q. Zhang, “Study of recycling blast furnace dust by magnetization roasting with straw charcoal as reductant,” Physicochem. Probl. Miner. Process., vol. 58, no. 3, pp. 149265, 2022. https://doi.org/10.37190/ppmp/149265 DOI: https://doi.org/10.37190/ppmp/149265
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2024 Iván Omar Acuña Gutiérrez, Damaris Margarita Puente Siller, José Manuel González de la Cruz, Luis Enrique Álvarez García, Juan Antonio López Corpus, Alberto Perea Garduño

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors who publish in this journal accept the following conditions:
The authors retain the copyright and assign to the journal the right of the first publication, with the work registered with the Creative Commons Attribution license 4.0, which allows third parties to use what is published as long as they mention the authorship of the work and the first publication in this magazine.
Authors may make other independent and additional contractual agreements for the non-exclusive distribution of the version of the article published in this journal (eg, include it in an institutional repository or publish it in a book) as long as they clearly indicate that the work it was first published in this magazine.
Authors are allowed and encouraged to share their work online (for example: in institutional repositories or personal web pages) before and during the manuscript submission process, as it can lead to productive exchanges, greater and more quick citation of published work (see The Effect of Open Access).











