Transformación de Plomo Potencialmente Tóxico a Pb (NO3)2 Cristalino
DOI:
https://doi.org/10.37636/recit.v23106112Palabras clave:
Plomo residual tóxico, Síntesis química, Producto nitrato de plomo.Resumen
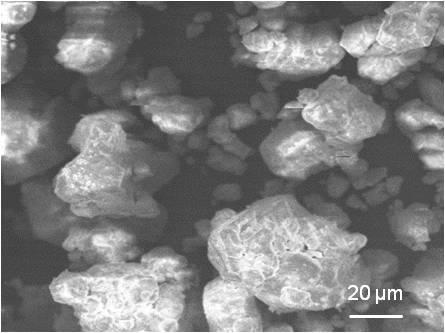
La acumulación de plomo en el ambiente es una causa de problemas de salud en humanos. La dosis letal media reportada en mg/kg para Pb, Pb (NO3)2 y PbO es de 400, 2250 y 2000, respectivamente. Debido a la alta toxicidad del plomo metálico con respecto al nitrato de plomo se ha desarrollado una metodología para transformar el plomo residual a nitrato de plomo, cuya toxicidad es cinco veces menor que el plomo metálico. Por otra parte, el nitrato de plomo puede ser precursor para la síntesis de materiales con potencial aplicación industrial. La síntesis del Pb (NO3)2 se realiza mezclando el plomo metálico residual y ácido nítrico 15.7 molar en una proporción de reactivos [1:4]. Posteriormente, la mezcla se somete a agitación magnética hasta disolver el plomo y formar un precipitado blanco, se seca a 110 °C durante 1 h obteniendo un rendimiento experimental del 99 %. El material sintetizado se caracteriza por difracción de rayos–X, microscopía electrónica de barrido y espectroscopia por dispersión de energía. Así mismo, esta metodología tiene un impacto favorable en los ecosistemas, ya que la contaminación por plomo se verá disminuida.Descargas
Citas
S. Tiwari, I. P. Tripathi, and H. L. Tiwari. "Effects of lead on Environment." International Journal of Emerging Research in Management &Technology vol. 2 no. 6, pp 1-5, 2013. http://scholar.google.com/citations?user=oNsCI8QAAAAJ&hl=en
D. Pavlov, Lead-Acid Batteries: Science and Technology, Great Britain, Elsevier, pp. 643, 2011. https://www.elsevier.com/books/lead-acid-batteries-science-and-technology/pavlov/978-0-444-59552-2
U.S. Geological Survey, Mineral commodity summaries 2016: U.S. Geological Survey, 2016. https://www.usgs.gov/centers/nmic/mineral-commodity-summaries
R. Zhang, V. L. Wilson, A. Hou and G. Meng, "Source of lead pollution, its influence on public health and the countermeasures", Int. J. of Health, Animal science and Food safety, vol. 2, no. 1, 18-31, 2015. https://doi.org/10.13130/2283-3927/4785
Q. Li, H. Cheng, T. Zhou, C. Lin and S. Guo, "The estimated atmospheric lead emissions in China, 1990-2009", Atmospheric Environment, vol. 60, pp. 1-8, 2012. https://doi.org/10.1016/j.atmosenv.2012.06.025 DOI: https://doi.org/10.1016/j.atmosenv.2012.06.025
S. Lin, X. Wang, I. T. S Yu, W. Tang, J. Miao, J. Li, S. Wu and X. Lin, "Environmental Lead Pollution and Elevated Blood Lead Levels Among Children in a Rural Area of China", Am J Public Health, vol. 101 no. 5, pp. 834-841, 2011. https://doi.org/10.2105/AJPH.2010.193656 DOI: https://doi.org/10.2105/AJPH.2010.193656
J.-P. Lucas, B. L. Bot, P. Glorennec, A. Etchevers, P. Bretine,F. Douay, V. Sébille, L. Bellanger, C. Mandin, "Lead contamination in French children's homes and environment", Environmental Research, vol. 116, pp. 58-65, 2012. https://doi.org/10.1016/j.envres.2012.04.005 DOI: https://doi.org/10.1016/j.envres.2012.04.005
S. Chao, J. LiQin and Z. WenJun. "A review on heavy metal contamination in the soil worldwide: Situation, impact and remediation techniques", Environmental Skeptics and Critics, vol. 3, no. 2, pp. 24-35, 2014. http://www.iaees.org/publications/journals/environsc/articles/2014-3%282%29/a-review-on-heavy-metal-contamination-in-the-soil-worldwide.pdf
K. Neeti and T. Prakash, "Effects of heavy metal poisoning during pregnancy." Int Res J Environment Sci, vol. 2 no. 1, pp. 88-92, 2013. https://www.karunaflame.com/karunaflame/wp-content/uploads/2016/11/Effects-of-Heavy-Metal-Poisoning-during-Pregnancy.pdf
H. Dapul and D. Laraque, "Lead poisoning in children." Advances in pediatrics, vol 61, no. 1, pp. 313-333, 2014.
https://doi.org/10.1016/j.yapd.2014.04.004 DOI: https://doi.org/10.1016/j.yapd.2014.04.004
M. P. Iqbal, "Lead pollution-a risk factor for cardiovascular disease in Asian developing countries." Pakistan journal of pharmaceutical sciences, vol. 25, no. 1, pp. 289-294, 2012. https://pdfs.semanticscholar.org/bfbe/e3af44f7be9cee8941612a356d4fc9c1d7d6.pdf
Wani, Ab Latif, Anjum Ara, and Jawed Ahmad Usmani. "Lead toxicity: a review." Interdisciplinary toxicology 8.2 (2015): 55-64. https://doi.org/10.1515/intox-2015-0009 DOI: https://doi.org/10.1515/intox-2015-0009
A.A. Hegazy, M.M. Zaher, M.A. Abd El-Hafez, A.A. Morsy, R.A. Saleh, "Relation between anemia and blood levels of lead, copper, zinc, and iron among children", BMC Res. Notes, vol. 3, pp. 133-141, 2010. https://doi.org/10.1186/1756-0500-3-133 DOI: https://doi.org/10.1186/1756-0500-3-133
A. Reuben, A. Caspi, D. W. Belsky, J. Broadbent, H. Harrington, K. Sugden, and T. E. Moffitt, "Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between childhood and adulthood." Jama, vol. 317, no.12, pp. 1244-1251, 2017.
https://doi.org/10.1001/jama.2017.1712 DOI: https://doi.org/10.1001/jama.2017.1712
N. H. Kim, Y. Y. Hyun, K. B. Lee, Y. Chang, S. Rhu, K Oh, and C. Ahn. "Environmental heavy metal exposure and chronic kidney disease in the general population." Journal of Korean medical science, vol. 30, no. 3, pp. 272-277, 2015. DOI: https://doi.org/10.3346/jkms.2015.30.3.272
https://doi.org/10.3346/jkms.2015.30.3.27
J. P. Harp, and D. Y. Han. "Pb neurotoxicity: neuropsychological effects of lead toxicity." BioMed research international, vol. 2014, pp. 1-8, 2014. https://doi.org/10.1155/2014/840547 DOI: https://doi.org/10.1155/2014/840547
H, Delile, J. Blichert-Toft, J. P Goiran, S. Keay, and F. Albarède, "Lead in ancient Rome's city waters." Proceedings of the National Academy of Sciences vol. 111, no. 18, pp. 6594-6599, 2014. https://doi.org/10.1073/pnas.1400097111 DOI: https://doi.org/10.1073/pnas.1400097111
O. Tarragó, M. J. Brown. "Case Studies in Environmental Medicine: Lead Toxicity". Agency for Toxic Substances and Disease Registry. Course: WB2832. CE Original Date: June 12, (2017). CE Expiration Date: June 2019. pp. 185, 2017. https://www.atsdr.cdc.gov/csem/lead/docs/CSEM-Lead_toxicity_508.pdf
O. C. Eneh, P. A. Akah. "Acute toxicity assessment of crude lead-extract from electronic waste materials in Nigeria" Afr. J. Biotechnol., vol. 11, no. 88, pp. 15430-15437, 2012. https://doi.org/10.5897/AJB12.2476 DOI: https://doi.org/10.5897/AJB12.2476
F. K. Apaydin, S. Kalender, H. Bas, F. Demir, and Y. Kalender, "Lead Nitrate Induced Testicular Toxicity in Diabetic and Non-Diabetic Rats: Protective Role of Sodium Selenite", Yusuf. Braz. Arch. Biol. Technol., vol. 58, no. 1, pp. 68-74, 2015.
https://doi.org/10.1590/S1516-8913201400025 DOI: https://doi.org/10.1590/S1516-8913201400025
Oxido de plomo, Efectos sobre la salud humana. Red de datos toxicolófigos de la Biblioteca Nacional de Medicina de EE. UU., sitio: https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@DOCNO+638 consultada en junio de 2019.
Y. Oulhote, A. Le Tertre, A. Etchevers, B. Le Bot, J. P. Lucas, C. Mandin and P. Glorennec, "Implications of different residential lead standards on children's blood lead levels in France: predictions based on a national cross-sectional survey." International journal of hygiene and environmental health, vol. 216, no. 6, pp. 743-750, 2013. https://doi.org/10.1016/j.ijheh.2013.02.007 DOI: https://doi.org/10.1016/j.ijheh.2013.02.007
P. Scherrer. "Bestimmung der Grosse und der Inneren Struktur von Kolloidteilchen Mittels Rontgenstrahlen, Nachrichten von der Gesellschaft der Wissenschaften, Gottingen", Mathematisch-Physikalische Klasse, vol. 2, pp. 98-100, 1918. https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=1547275
A. L. Patterson, "The Scherrer Formula for X-Ray Particle Size Determination", Physical Review, vol. 56, pp. 978-982, 1939.
https://doi.org/10.1103/PhysRev.56.978 DOI: https://doi.org/10.1103/PhysRev.56.978
Joint Committee on Powder Diffraction Standards (JCPDS®)-International Centre for Diffraction Data (ICDD®). Powder Diffraction File TM No.: 65-2873 (Pb), 36-1462 (Pb(NO3)2), 05-0561 (α-PbO), 88-1589 (β-PbO) and 19-0697 (Pb12O19). Newtown Square, PA, USA. (2018). https://www.icdd.com/pdfsearch/
G. Zschornack, Handbook of X-Ray Data, Springer Berlin Heidelberg New York, pp. 969, 2007. https://www.springer.com/productFlyer_978-3-540-28618-9.pdf?SGWID=0-0-1297-72038922-0
Publicado
Cómo citar
Número
Sección
Categorías
Licencia
Derechos de autor 2019 Ulises Alejandro Villalón López, María Guadalupe Moreno Armenta, Arturo Barrera Rodríguez, Eduardo Rogel Hernández, Juan Manuel Quintana Melgoza

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Los autores/as que publiquen en esta revista aceptan las siguientes condiciones:
- Los autores/as conservan los derechos de autor y ceden a la revista el derecho de la primera publicación, con el trabajo registrado con la licencia de atribución de Creative Commons 4.0, que permite a terceros utilizar lo publicado siempre que mencionen la autoría del trabajo y a la primera publicación en esta revista.
- Los autores/as pueden realizar otros acuerdos contractuales independientes y adicionales para la distribución no exclusiva de la versión del artículo publicado en esta revista (p. ej., incluirlo en un repositorio institucional o publicarlo en un libro) siempre que indiquen claramente que el trabajo se publicó por primera vez en esta revista.
- Se permite y recomienda a los autores/as a compartir su trabajo en línea (por ejemplo: en repositorios institucionales o páginas web personales) antes y durante el proceso de envío del manuscrito, ya que puede conducir a intercambios productivos, a una mayor y más rápida citación del trabajo publicado (vea The Effect of Open Access).











