Estudio DFT a moléculas derivadas de benzimidazol y piridina con capacidad inhibidora de corrosión
DOI:
https://doi.org/10.37636/recit.v211419Palabras clave:
Inhibidores, DFT, Heterocíclicos, Piridina, Benzimidazol, Corrosión.Resumen
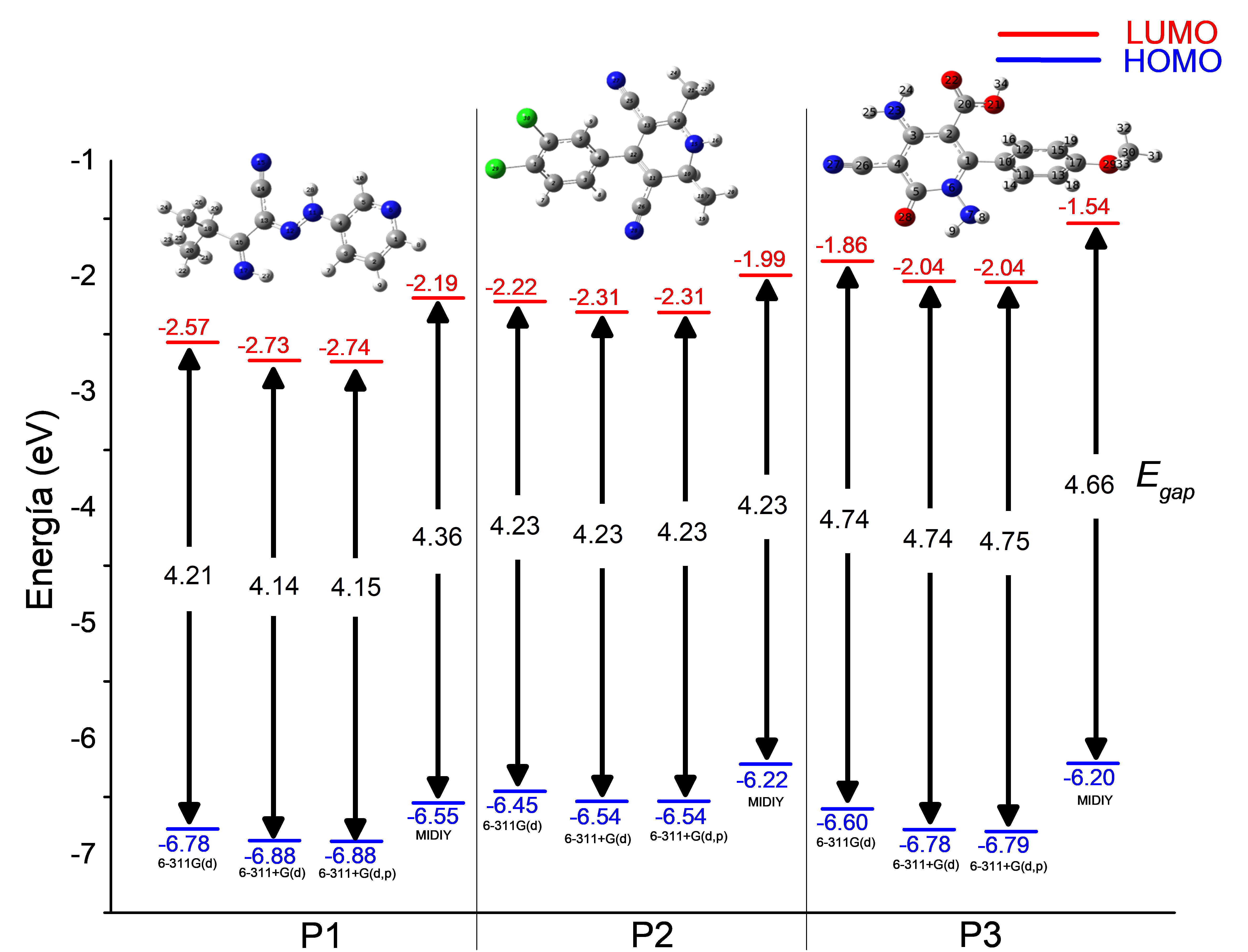
Los inhibidores basados en heteroátomos de nitrógeno han mostrado ser uno de los productos químicos eficaces en la inhibición de la corrosión de metales. Este estudio fue llevado a cabo con la teoría de funcionales de la densidad (DFT), utilizando distintos niveles de cálculo. Diversas propuestas fueron evaluadas para estimar los niveles de energía de los orbitales moleculares de frontera (HOMO-LUMO) y su isodensidad. Dichos parámetros fueron considerados para determinar la parte nucleofílica y electrofílica de las moléculas. Finalmente, se llevó a cabo un análisis de la reactividad química con los parámetros de afinidad electrónica, el potencial de ionización, la dureza química y el índice de electrofilicidad; el objetivo fue determinar el efecto sobre la dureza química al cambiar la posición de los sustituyentes.Descargas
Citas
I. B. Obot and A. Madhankumar, "Synergistic effect of iodide ion addition on the inhibition of mild steel corrosion in 1 M HCl by 3-amino-2-methylbenzylalcohol," Mater. Chem. Phys., vol. 177, pp. 266-275, Jul. 2016. https://doi.org/10.1016/j.matchemphys.2016.04.027 DOI: https://doi.org/10.1016/j.matchemphys.2016.04.027
M. Lashgari, M. R. Arshadi, G. a Parsafar, and V. S. Sastri, "Cluster/Polarized Continuum Models for Density Functional Theory Investigations of Benzimidazole Corrosion Inhibitors at Metal/Solution Interface," Corrosion, vol. 62, no. 3, pp. 199-206, Mar. 2006. https://doi.org/10.5006/1.3278266 DOI: https://doi.org/10.5006/1.3278266
E. Garcia-Ochoa, S. J. Guzmán-Jiménez, J. G. Hernández, T. Pandiyan, J. M. Vásquez-Pérez, and J. Cruz-Borbolla, "Benzimidazole ligands in the corrosion inhibition for carbon steel in acid medium: DFT study of its interaction on Fe30 surface," J. Mol. Struct., vol. 1119, pp. 314-324, Sep. 2016. https://doi.org/10.1016/j.molstruc.2016.04.057 DOI: https://doi.org/10.1016/j.molstruc.2016.04.057
O. Krim, A. Elidrissi, B. Hammouti, A. Ouslim, and M. Benkaddour, "Synthesis, characterization, and comparative study of pyridine derivatives as corrosion inhibitors of mild steel in hcl medium," chem. eng. commun., vol. 196, no. 12, pp. 1536-1546, aug. 2009. https://doi.org/10.1080/00986440903155451 DOI: https://doi.org/10.1080/00986440903155451
R. Peverati and D. G. Truhlar, "M11-L: A Local Density Functional That Provides Improved Accuracy for Electronic Structure Calculations in Chemistry and Physics," J. Phys. Chem. Lett., vol. 3, no. 1, pp. 117-124, Jan. 2012. https://doi.org/10.1021/jz201525m DOI: https://doi.org/10.1021/jz201525m
C. Adamo and V. Barone, "Toward reliable density functional methods without adjustable parameters: The PBE0 model," J. Chem. Phys., vol. 110, no. 13, p. 6158, 1999. https://doi.org/10.1063/1.478522 DOI: https://doi.org/10.1063/1.478522
R. Peverati and D. G. Truhlar, "Screened-exchange density functionals with broad accuracy for chemistry and solid-state physics," Phys. Chem. Chem. Phys., vol. 14, no. 47, p. 16187, 2012. https://doi.org/10.1039/c2cp42576a DOI: https://doi.org/10.1039/c2cp42576a
Y. Zhao and D. G. Truhlar, "The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other function," Theor. Chem. Acc., vol. 120, no. 1-3, pp. 215-241, May 2008. https://doi.org/10.1007/s00214-007-0310-x DOI: https://doi.org/10.1007/s00214-007-0310-x
L. A. Curtiss, P. C. Redfern, V. Rassolov, G. Kedziora, and J. A. Pople, "Extension of Gaussian-3 theory to molecules containing third-row atoms K, Ca, Ga-Kr," J. Chem. Phys., vol. 114, no. 21, p. 9287, 2001. https://doi.org/10.1063/1.1366337 DOI: https://doi.org/10.1063/1.1366337
C. J. Cramer, Essentials of Computational Chemistry: theories and models, 2nd ed. John Wiley & Sons, Inc., 2013. https://www.wiley.com/en-us/Essentials+of+Computational+Chemistry%3A+Theories+and+Models%2C+2nd+Edition-p-9780470091821
R. G. Parr and R. G. Pearson, "Absolute hardness: companion parameter to absolute electronegativity," J. Am. Chem. Soc., vol. 105, no. 26, pp. 7512-7516, Dec. 1983. https://doi.org/10.1021/ja00364a005 DOI: https://doi.org/10.1021/ja00364a005
J. L. Gázquez, A. Cedillo, and A. Vela, "Electrodonating and Electroaccepting Powers," J. Phys. Chem. A, vol. 111, no. 10, pp. 1966-1970, Mar. 2007. https://doi.org/10.1021/jp065459f DOI: https://doi.org/10.1021/jp065459f
R. G. Parr, L. v. Szentpály, and S. Liu, "Electrophilicity Index," J. Am. Chem. Soc., vol. 121, no. 9, pp. 1922-1924, Mar. 1999. https://doi.org/10.1021/ja983494x DOI: https://doi.org/10.1021/ja983494x
J. Cruz, R. Martínez, J. Genesca, and E. García-Ochoa, "Experimental and theoretical study of 1-(2-ethylamino)-2-methylimidazoline as an inhibitor of carbon steel corrosion in acid media," J. Electroanal. Chem., vol. 566, no. 1, pp. 111-121, 2004. https://doi.org/10.1016/j.jelechem.2003.11.018 DOI: https://doi.org/10.1016/j.jelechem.2003.11.018
D. Turcio-Ortega, T. Pandiyan, J. Cruz, and E. Garcia-Ochoa, "Interaction of Imidazoline Compounds with Fe n ( n = 1−4 Atoms) as a Model for Corrosion Inhibition: DFT and Electrochemical Studies," J. Phys. Chem. C, vol. 111, no. 27, pp. 9853-9866, Jul. 2007. https://doi.org/10.1021/jp0711038 DOI: https://doi.org/10.1021/jp0711038
T. M. McCormick et al., "Conjugated polymers: Evaluating DFT methods for more accurate orbital energy modeling," Macromolecules, vol. 46, pp. 3879-3886, 2013. https://doi.org/10.1021/ma4005023 DOI: https://doi.org/10.1021/ma4005023
R. V. Solomon, A. P. Bella, S. A. Vedha, and P. Venuvanalingam, "Designing benzosiloles for better optoelectronic properties using DFT & TDDFT approaches.," Phys. Chem. Chem. Phys., vol. 14, no. 41, pp. 14229-37, 2012. https://doi.org/10.1039/c2cp41554b DOI: https://doi.org/10.1039/c2cp41554b
U. Salzner, J. B. Lagowski, P. G. Pickup, and R. a. Poirier, "Comparison of geometries and electronic structures of polyacetylene, polyborole, polycyclopentadiene, polypyrrole, polyfuran, polysilole, polyphosphole, polythiophene, polyselenophene and polytellurophene," Synth. Met., vol. 96, no. 3, pp. 177-189, Aug. 1998. https://doi.org/10.1016/S0379-6779(98)00084-8 DOI: https://doi.org/10.1016/S0379-6779(98)00084-8
M. J. Bahrami, S. M. A. Hosseini, and P. Pilvar, "Experimental and theoretical investigation of organic compounds as inhibitors for mild steel corrosion in sulfuric acid medium," Corros. Sci., vol. 52, no. 9, pp. 2793-2803, Sep. 2010. https://doi.org/10.1016/j.corsci.2010.04.024 DOI: https://doi.org/10.1016/j.corsci.2010.04.024
S. Kaya, B. Tüzün, C. Kaya, and I. B. Obot, "Determination of corrosion inhibition effects of amino acids: Quantum chemical and molecular dynamic simulation study," J. Taiwan Inst. Chem. Eng., vol. 58, pp. 528-535, Jan. 2016. https://doi.org/10.1016/j.jtice.2015.06.009 DOI: https://doi.org/10.1016/j.jtice.2015.06.009
Publicado
Cómo citar
Número
Sección
Categorías
Licencia
Derechos de autor 2019 Jorge Reyes-Corrales, Rody Soto-Rojo, Daniel Glossman-Miknit, Jesús Baldenebro-López

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Los autores/as que publiquen en esta revista aceptan las siguientes condiciones:
- Los autores/as conservan los derechos de autor y ceden a la revista el derecho de la primera publicación, con el trabajo registrado con la licencia de atribución de Creative Commons 4.0, que permite a terceros utilizar lo publicado siempre que mencionen la autoría del trabajo y a la primera publicación en esta revista.
- Los autores/as pueden realizar otros acuerdos contractuales independientes y adicionales para la distribución no exclusiva de la versión del artículo publicado en esta revista (p. ej., incluirlo en un repositorio institucional o publicarlo en un libro) siempre que indiquen claramente que el trabajo se publicó por primera vez en esta revista.
- Se permite y recomienda a los autores/as a compartir su trabajo en línea (por ejemplo: en repositorios institucionales o páginas web personales) antes y durante el proceso de envío del manuscrito, ya que puede conducir a intercambios productivos, a una mayor y más rápida citación del trabajo publicado (vea The Effect of Open Access).











